The study found that senescent cells are integral components of the skeletal muscle regenerative niche and repress regeneration at all stages of life. By using single-cell transcriptomics and a senescent-cell enrichment sorting protocol, the researchers were able to identify and isolate different senescent cell types from damaged muscles of young and old mice. They discovered that senescent cells create an aged-like inflamed niche that arrests stem cell proliferation and regeneration, and that reducing the burden of senescent cells or their inflammatory secretome accelerates regeneration in young and old mice. The study also found that transplantation of senescent cells delays regeneration. These findings may open potential paths for improving muscle repair throughout life.
- This new study explores the impact of cellular senescence and the senescence-associated secretory phenotype (SASP) on tissue regeneration.
- The researchers use intestinal organoids as a model and find that SASP factors released by senescent fibroblasts negatively impact stem cell activity and differentiation, ultimately impairing crypt formation.
- They identify the secreted N-terminal domain of Ptk7 as a key component of SASP that affects stem cell behavior through non-canonical Wnt/Ca2+ signaling.
- Ptk7 changes the cytosolic calcium levels, which leads to nuclear translocation of YAP and expression of YAP/TEAD target genes, impairing stem cell differentiation and symmetry breaking.
- The study concludes by revealing secreted Ptk7 as a factor released by senescent cells that contributes to tissue dysfunction in aging and disease.
Here is another paper discusses the role of transposable elements with viral origins, such as endogenous retroviruses (ERVs), in aging.
The authors found that in human senescent cells, HERVK (HML-2) ERVs are activated and produce retrovirus-like particles (RVLPs).
These RVLPs can cause senescence phenotypes in young cells, which can be blocked by neutralizing antibodies.
The activation of ERVs was also found in aged primates, mice, and human cells.
Repression of ERVs alleviates cellular senescence and tissue degeneration and slows down organismal aging.
The authors suggest that the activation of ERVs is a hallmark and driving force of cellular senescence and tissue aging.
The new study investigated the relationship between senescent hepatocytes, regeneration, and secreted factors on liver tumor development. They reported the following points:
- Liver damage and regeneration triggered immunosurveillance escape of premalignant hepatocytes, leading to tumor development.
- TFF3 was overexpressed in premalignant hepatocytes and liver cancer, and TFF3 deficiency reduced tumor development.
- TFF3 deficiency attenuated malignant transformation by increasing IGFBP5 expression, which dampened IGF receptor signaling.
- The findings shed light on the early events of liver cancer and the roles of TFF3 and IGFBP5 in tumorigenesis
twelve hallmarks of aging:
genomic instability,
telomere attrition,
epigenetic alterations,
loss of proteostasis,
disabled macroautophagy,
deregulated nutrient-sensing,
mitochondrial dysfunction,
cellular senescence,
stem cell exhaustion,
altered intercellular communication,
chronic inflammation,
dysbiosis.
Cell. 2023 Jan 19;186(2):243-278. doi: 10.1016/j.cell.2022.11.001. Epub 2023 Jan 3.
Findings from the new studies:
- Lymphatic vessels in bone expand during genotoxic stress.
- The growth of lymphatic vessels in bones is driven by the signaling between VEGF-C/VEGFR-3 and IL6 in response to genotoxic stress.
- During lymphangiogenesis, the CXCL12 protein secreted by lymphatic endothelial cells plays a critical role in bone and blood cell regeneration.
- The expansion of Myh11+ CXCR4+ pericytes, which contribute to bone and blood cell regeneration, is triggered by CXCL12.
- In aged animals, the growth of lymphatic vessels and Myh11-positive cells in response to genotoxic stress is impaired.
- These findings suggest that lymphangiogenesis could be used as a therapeutic approach to stimulate bone and blood cell regeneration.
Another interesting study:
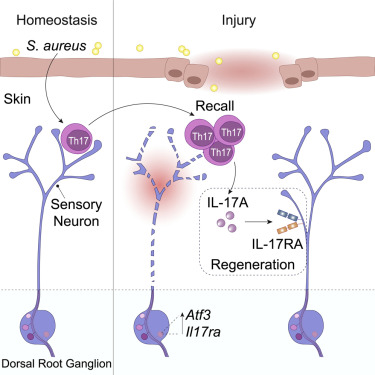
- Tissue-resident commensal-specific T cells and sensory nerve fibers are found together in the dermis, expressing a transcriptional program associated with neuronal interaction and repair, and promoting axon growth and local nerve regeneration.
- Interleukin-17A (IL-17A) cytokine released by commensal-specific Th17 cells upon injury signals to sensory neurons via IL-17 receptor A, which is specifically upregulated in injured neurons.
- Preemptive immunity to the microbiota can rapidly bridge biological systems by directly promoting neuronal repair, and IL-17A is a major determinant of this process.
- A new study has shown that a combination of factors called Yamanaka factors (OCT4, SOX2, and KLF4 or OSK) can reverse age-related changes in cells.
- Researchers found that delivering OSK to old mice extended their remaining lifespan by 109%.
- The OSK treatment also improved the mice’s health, including their frailty score.
- In human keratinocytes, OSK had positive effects on aging markers, potentially restoring a more youthful state.
- Researchers used single-cell transcriptomics and analysis to study activated cell states in regenerating zebrafish hearts
- Fibroblast-like cell states emerged after injury, and collagen-12-expressing fibroblasts were found to have proregenerative function
- Activated fibroblasts were found to come from two sources: the epicardium and endocardium
- Wnt signaling was found to regulate the endocardial fibroblast response
- These findings could lead to new approaches to modulate the regenerative capacity of the vertebrate heart.
- Aging involves biological changes that increase vulnerability to stressors, chronic diseases (e.g. diabetes, cardiovascular disease, cancer, neurodegeneration), and functional decline.
- Compromised autophagy (the process of recycling obsolete cell components) contributes to the accumulation of intracellular protein aggregates, damaged mitochondria, and lipofuscin deposits during aging.
- Mitophagy, a specific autophagy pathway that selectively targets mitochondria, has been identified as playing a role in sarcopenia (decline in muscle mass and strength) and age-related muscle dysfunction.
- Perturbations in mitochondrial quality control processes have been linked to mtDNA instability, iron homeostasis perturbations, and neurodegeneration.
- Upregulation of autophagy via pharmacological agents has been shown to promote the clearance of protein aggregates and alleviate disease phenotypes in vitro and in vivo in ALS.
- The p.K126R RAB7 mutation responsible for Charcot–Marie–Tooth type 2B disease, a dominant axonal peripheral neuropathy, leads to an increase in lysosomal activity and autophagy associated with a motor phenotype.
- Rubicon, a protein involved in autophagy inhibition, is implicated in the amyloid pathway and neurodegeneration and is a potential therapeutic target for Alzheimer’s disease and other neurodegenerative disorders.
- Decline in neuronal autophagy is implicated in determining lifespan and healthspan.
New studies showed:
1 Mammalian telomeres consist of (TTAGGG)n repeats. Transcription of the C-rich strand generates a G-rich RNA, termed TERRA
2 The translation of TERRA would generate two dipeptide repeat proteins = valine–arginine (VR)n and glycine–leucine (GL)n
3 Both VR and GL form long filaments with amyloid properties
4 The telomere dysfunction dysfunction may express two dipeptide repeat proteins
- Cellular senescence is a stress response that activates innate immune cells.
- Senescent cells have features that make them efficient in activating dendritic cells and antigen-specific CD8 T cells.
- Immunization with senescent cancer cells elicits strong antitumor protection mediated by DCs and CD8 T cells.
- Protection provided by senescent cancer cells is better than immunization with cancer cells undergoing immunogenic cell death.
- Induction of senescence in human primary cancer cells enhances their ability to activate autologous antigen-specific tumor-infiltrating CD8 lymphocytes.
- Senescent cancer cells can be used to develop efficient and protective CD8-dependent antitumor immune responses.
A couple of important points on MSCs:
1 MSCs are fibroblast-like multipotent progenitor cells with immunosuppressive properties
2 adipose tissue-derived MSCs (ASCs) generally inhibit CD4+ T cell proliferation; ASCs from ankylosing spondylitis patients could enhance the generation of anti-inflammatory regulatory T cells (Treg) and could downregulate interferon γ expression under co-culturing with peripheral blood mononuclear cells
a loss of epigenetic information is a reversible cause of aging
- Acute lipid accumulation in the liver induces the phosphorylation of EIF2S1 (eukaryotic translation initiation factor 2), which subsequently attenuates Mier1 translation.
- The downregulation of MIER1 promotes cell cycle gene expression and regeneration through chromatin remodeling.
Recently, stem cell-based spheroids have been utilized in tissue engineering and regenerative medicine as transplantable treatments targeting bone, cartilage, tendon, muscle, dental, nerve, blood vessel, and skin tissue.
An inflammatory process (low grade, clinically not perceptible, chronic, systemic) occurring in the absence of infection that is a fundamental attribute of aging
Senescence-associated secretory phenotype (SASP) is comprised largely of pro-inflammatory, extracellular matrix-degrading, complement-activating and pro-coagulatory factors secreted by senescent cells.
- Biliary epithelial cells (BECs) as potential functional hepatocyte transdifferentiation source
- Transitional Liver Progenitor Cell (TLPC) originates from BECs, and may differentiates into hepatocytes during regeneration from severe liver injury
- TLPCs are bipotent, as they either differentiate into hepatocytes or re-adopt BEC fate.
- Notch signaling orchestrates BEC-to-TLPC conversion, Wnt/β-catenin signaling orchestrates TLPC-to-hepatocyte conversion
- Yeast cells have a transcriptional toggle switch that causes them to die by one of two fates: nucleolar decline or mitochondrial decay.
- Zhou et al. rewired this transcriptional switch into a negative-feedback loop to cause yeast cells to oscillate between the two states.
- The oscillations resulted in an 82% increase in cellular lifespan.
- This study represents a step forward in the use of engineering principles to design synthetic gene circuits that control complex biological traits.
- The rewiring of the endogenous toggle switch created an autonomous genetic clock that generated sustained oscillations between nucleolar and mitochondrial aging processes in individual cells.
- The oscillations increased cellular lifespan by delaying the commitment to aging caused by either the loss of chromatin silencing or the depletion of heme.